Clinical History
None Available.
Clinical history:
None available
What is the most likely diagnosis?
- Acute allergic interstitial nephritis
- Mesangioproliferative GN associated with HCV
- Heroin-induced nephropathy
- HIV associated nephropathy
- Hemolytic-uremic syndrome
What is the most common renal parenchymal disease seen on biopsy for this condition?
- Focal segmental glomerulosclerosis
- Acute tubular necrosis
- Nodular glomerulosclerosis
- Benign nephrosclerosis
- Membranous glomerulonephritis
Which of the following distinguishes this condition best from other similar kidney diseases?
- Homogeneous echogenicity
- Enlarged kidney
- Increased corticomedullary definition
- Expansion of renal sinus fat
Which of the following clinical factors is characteristic of this disease?
- Nephritic range proteinuria
- Edema
- Hypogammaglobulinemia
- Low viral load
- Normotension
Answers:
- HIV associated nephropathy
- FSGS
- Enlarged kidneys
- Normotension
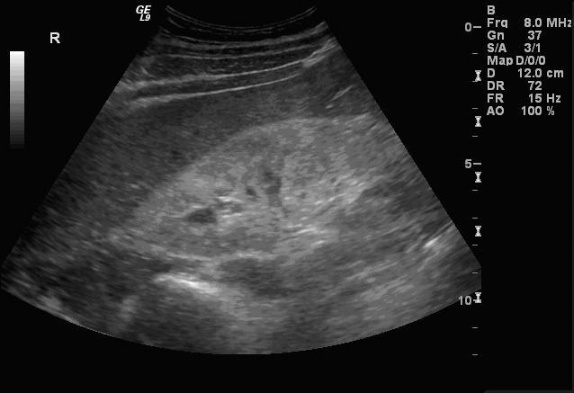
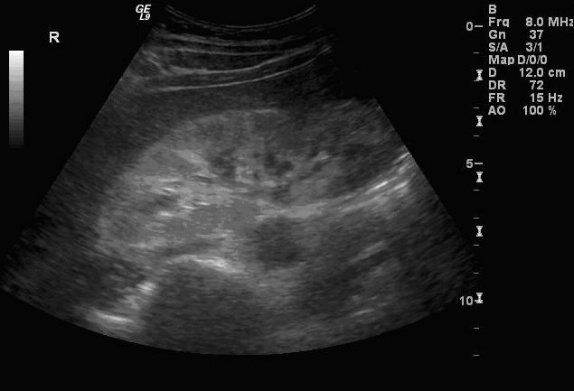
Sonographic Findings:
HIV-associated nephropathy (HIVAN) commonly presents with the following sonographic findings: Enlarged, globular kidneys with marked echogenicity in a heterogeneous pattern, loss of corticomedullary definition, and obliteration of renal sinus fat. These findings may be more prominent in setting of clinical AIDS. HIV-positive patients may have HIVAN according to diagnostic criteria but still have few if any of the classic sonographic signs. According to the study by Di Fiori et al., only 20% of HIVAN patients present with enlarged kidneys and only 38% with decrease of corticomedullary definition. Increased echogenicity (greater than liver and spleen) and a heterogeneous echopattern were the most common findings; these are seen in ~75% of cases. Globular morphology and loss of renal sinus fat were found in about half of cases. These classic findings are typically more apparent with more severe disease.
The increased echogenicity associated with HIVAN often presents in characteristic patterns. Diffuse echogenicity is seen, but bands or patchy areas are more common. The latter pattern may be striking – bands of increased echogenicity may extend from the central kidney to the cortex.
Pathology:
The diagnostic pentad includes proteinuria, azotemia, normal to enlarged kidneys by US, absence of HTN, and focal segmental glomerulosclerosis on biopsy. Biopsy is a somewhat controversial topic, and it has been argued that it increases morbidity without improving long-term outcome. FSGS is a sensitive but not a specific finding for HIVAN, and may also be due to IgA nephropathy, cryoglobulinemia, amyloidosis, and lupus-like immune complex glomerulopathy. FSGS is also a common histologic feature of heroin-associated nephropathy, and should be considered when IV drug use is suspected.
Etiology:
Multiple influences are implicated in the development of HIVAN. Factors range from a direct viral infection to side effects of HIV medications. Glomerular and tubular epithelial cells have been shown to be selectively infected by HIV-1, suggesting a renal viral reservoir. Azotemia and lack of hypertension may be due to salt wasting, volume depletion, and poor nutrition in AIDS patients. The echogenicity and enlargement of the kidney is presumably due to prominent interstitial expansion by cellular infiltration and markedly dilated tubules containing echogenic, proteinaceous casts.
Epidemiology:
One percent of new end-stage renal disease cases in the US are due to HIVAN. Most patients are young black males, and half are IV drug addicts. The male to female ratio is pronounced at 10:1.
Presentation:
Nephrotic range proteinuria is the most common presenting sign; HIVAN should be considered in the setting of proteinuria and HIV positivity. Other typical lab findings include azotemia, hypoalbuminemia, and hyperlipidemia. Hypertension and edema are rare presenting signs in spite of renal disease and protein loss. The absence of edema may be due to the salt-losing and high oncotic pressures attributed to hypergammaglobulinemia.
Intervention:
Dialysis has not been shown to improve survival. Biopsy is of uncertain value. Clinical suspicion alone is insensitive for diagnosis, and some authors recommend obtaining biopsy when daily protein excretion exceeds 1g. Others state that biopsy adds to morbidity and mortality as well as cost without significantly affecting patient outcome or treatment, making biopsy inappropriate.
Natural History:
HIVAN is a common complication among HIV patients, and the US findings may evolve as part of the natural history of the disease. Clinical AIDS is a major negative prognostic indicator, especially when the CD4 count is less than 50 cells/uL. The presence of parenchymal inhomogeneity, decreased visibility of renal sinus fat, and decreased cortiomedullary definition are, correspondingly, associated with poorer outcomes. Prior to HAART, mortality with a diagnosis of HIVAN was 100% within 6 months. With the advent of newer therapies, survival has greatly improved. For most patients, opportunistic infection rather than renal disease will be the cause of death.
Ryan Alexander, MD
PGY1, University of Pittsburgh School of Medicine
Department of Radiology
References:
- Atta MG, Lucas GM, Fine DM. HIV-associated nephropathy: epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther. 2008 Jun;6(3):365-71. Review.
- Post FA, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008 Apr 15;46(8):1282
- Wyatt CM, Rosenstiel PE, Klotman PE. HIV-associated nephropathy. Contrib Nephrol. 2008;159:151-61.
- Fine DM, Atta MG. Kidney disease in the HIV-infected patient. AIDS Patient Care STDS. 2007 Nov;21(11):813-24. Review.
- Anochie IC, Eke FU, Okpere AN. Human immunodeficiency virus-associated nephropathy (HIVAN) in Nigerian children. Pediatr Nephrol. 2008 Jan;23(1):117-22. Epub 2007 Nov 6.