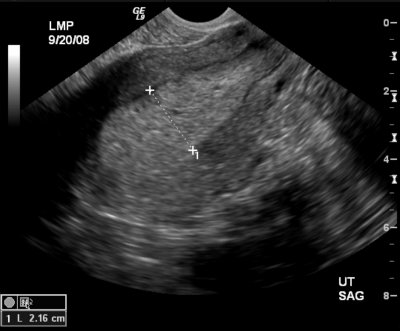
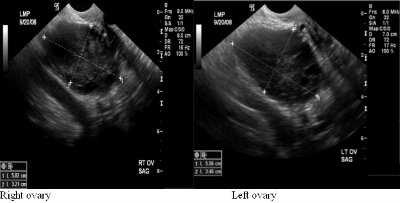
Clinical history:
37-year-old female with elevated androgen levels and hirsutism; LMP was thirteen days prior to time of exam.
Up to what value is the normal thickness of the endometrial echo complex in premenopausal women? What about postmenopausal women?
- 5 mm
- 10 mm
- 15 mm
- 20 mm
True or False: A diagnosis of endometrial hyperplasia can be made on the above imaging findings alone.
Given the imaging findings above with consideration of the clinical hirsutism and elevated androgen levels, what is the most likely etiology of the patient’s endometrial thickening?
- Retained products of conception
- Serous ovarian cystadenoma
- Polycystic ovarian syndrome
- Pheochromocytoma
- 15 mm; 5 mm
- False
- Polycystic Ovarian Syndrome
Discussion:
The thickness of the endometrial echo complex on ultrasound evaluation is considered normal up to 1.5cm in premenopausal women and up to 5mm in postmenopausal women. 1 When the thickness exceeds these values, further workup with endometrial biopsy is warranted to differentiate between endometrial hyperplasia versus endometrial carcinoma. Histologic diagnosis is also important in the setting of endometrial hyperplasia as certain subtypes progress to endometrial carcinoma and therefore appropriate treatment with surveillance is needed. Endometrial hyperplasia is defined as the proliferation of endometrial glands. This occurs as a result of unopposed estrogen. Risk factors can include estrogen hormone replacement therapy, the use of Tamoxifen, anovulatory menstrual cycles, obesity, diabetes, PCOS, nulliparity, and estrogen producing tumors such as granulosa cell tumors and thecomas. 7 Gland proliferation can be classified as adenomatous versus cystic. In adenomatous hyperplasia the endometrial glands are closely spaced together as there is little stromal tissue. Cystic hyperplasia is resultant of cystic dilation of the glands with a greater proportion of stroma. 7 Further histologic classification subcategorizes endometrial hyperplasia into hyperplasia with and without atypia. Studies have shown that diagnoses without atypia have less than a 2% risk of developing endometrial carcinoma whereas diagnoses with atypia have a 25% risk of further development to endometrial carcinoma.1 Another study reported an 8% risk of carcinoma in patients without atypia and up to 52% with atypia. 5 Therefore, the most definitive therapy for patients with atypical cells found on biopsy is hysterectomy. For women of childbearing age, progestin therapy with an endometrial biopsy every three months until a hysterectomy is performed is offered. For patients of childbearing age without atypia, progestin therapy with a biopsy every three months is performed. After twelve months of normal biopsy results, frequency can be decreased to a yearly endometrial biopsy.1 Given the sonographic findings above, the patient’s endometrial thickening is most likely secondary to polycystic ovarian syndrome. Polycystic ovarian syndrome is defined as chronic anovulation and hyperandrogenism. Manifestations of this syndrome can include hirsutism, acne, obesity, infertility, and hair loss. The direct development of diabetes and cardiovascular disease secondary to PCOS is widely debated.6 Sonographic findings of PCOS have been defined as the presence of either 12 or more follicles measuring 2-9 mm in diameter or an ovarian volume greater than 10cm3.2 A thickened endometrium is also a common finding. The endometrium can have either a homogenous or heterogeneous appearance. One study reports that an endometrium with a heterogeneous appearance and small cysts is a sign of endometrial hyperplasia in 40% of patients with PCOS while the presence of endometrial hyperplasia was never found in the presence of a homogenous endometrium.6 Neha Desai Choksi MD, R2 University of Pittsburgh Medical CenterReferences:
- Ahuja, Anil T. Diagnostic Imaging: Ultrasound; 2007. Amirsys: 9:32-9:35.
- Balen Adam H. Ultrasound assessment of the polycystic ovary: international consensus definitions. Human Reproduction Update. 2003:9; 505-514.
- Davis, Patricia C., O’neill, Mary Jane et al. Sonohysterographic Findings of Endometrial and Subendometrial Conditions. Radiographics. July 2002;22: 809-811.
- Middleton W, Alfred K, Hertzberg B. The Requisites: Ultrasound, second edition; 2004. Mosby: 532-541.
- Miller, Caela, Pulcini, Joseph P et al. The ability of endometrial biopsies with atypical complex hyperplasia to guide surgical management. American Journal of Obstetrics and Gynecology. July 2008: 199; 69e1-69e4.
- Nagamani, Peri, Levine, Deborah. Sonographic Evaluation of the Endometrium in Patients With a History or an Appearance of Polycystic Ovarian Syndrome. Journal of Ultrasound in Medicine. July 2006: 26:55-58.
- Rumack, Carol M, Wilson, Stephanie R et al. Diagnostic Ultrasound, second edition; 1998. Mosby: 536-537.
- Setji, Tracy L. Polycystic Ovarian Syndrome: Diagnosis and Treatment. The American Journal of Medicine. February 2007: 120:22; 128-132.